Medical Devices
ExoVasc® PEARS
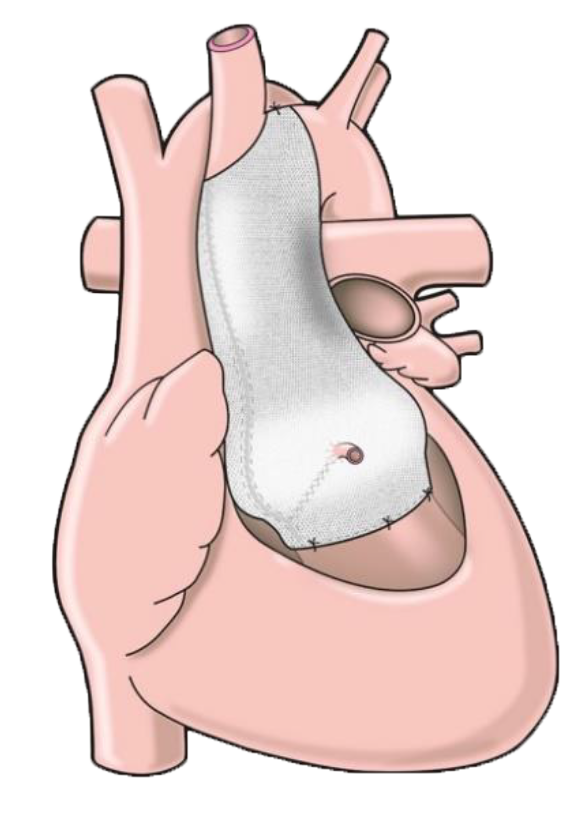
The ExoVasc® PEARS is used to support the aortic root and the ascending aorta. It is used in two procedures, which we refer to as Aortic PEARS and Ross-PEARS.
Before either procedure, the patient receives a CT scan to produce images and data from which a 3-dimensional model of the aorta is made. From this model, 3-D printing is used to create a polymeric former that exactly matches the patient’s individual aorta.
This former is used to provide the shape of the ExoVasc® PEARS implant, which is manufactured using polyethylene terephthalate, a soft and pliable biocompatible textile.
Once implanted, the ExoVasc® PEARS becomes incorporated into the aortic wall.
Further surgery to manage the condition for which it was implanted is unlikely to be necessary. However, the ExoVasc® PEARS does not preclude further aortic intervention if it is needed in the future.
It is not electrically conductive or magnetic and is compatible with CT, MRI body scanners and airport security scanners.
It requires no maintenance and will last for the patient’s lifetime.