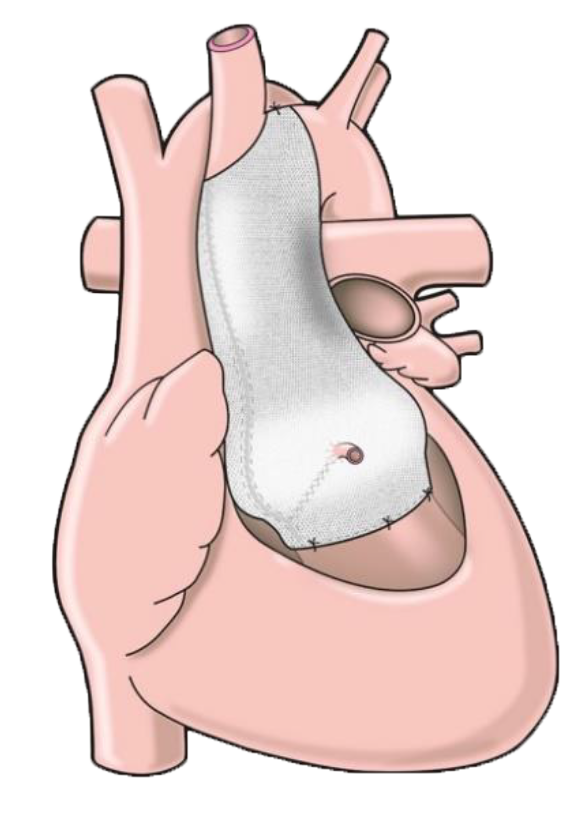
ExoVasc® PEARS is approved for sale in the UK, EU, Australia, New Zealand and Singapore.
Clinical trial information
The ExoVasc® PEARS was the subject of a clinical trial that was approved by the Local Research & Ethics Committee of the Royal Brompton & Harefield NHS Foundation Trust and the UK Medicines and Healthcare products Regulatory Agency (MHRA) – the government agency that is responsible for ensuring that medicines and medical devices are safe and effective.
In January 2010 the procedure received Clinical Practice Committee approval from the Royal Brompton & Harefield NHS Foundation Trust.
Quality Management System approvals
ISO 13485:2016 Medical Devices. Quality Management System. Requirements for Regulatory Purposes
The ExoVasc® PEARS is a custom-made device that is designed and manufactured by Exstent Limited to meet the requirements of each patient. The company operates a quality system that meets the requirements of ISO 13485:2016 for the design, development and manufacture of custom-made implantable medical devices used to provide external support to the ascending aorta for use by cardiothoracic surgeons.
MDSAP ISO 13485:2016
Exstent’s Quality Management System meets the requirements of MDSAP (Medical Device Single Audit Programme) ISO 13485:2016 including country-specific requirements for Australia, USA, Canada, Brazil and Japan for the design and development, and manufacture of custom-made implantable vascular prostheses used to provide external support to the ascending aorta for use by cardiothoracic surgeons.
MHRA, United Kingdom
The ExoVasc® PEARS is registered with MHRA as a custom-made device in accordance with The Medical Devices Regulations 2002.
NICE approval, United Kingdom
The procedure was submitted to the Interventional Procedures Advisory Committee (IPAC) of the National Institute for Health and Clinical Excellence (NICE).
NICE issued guidance to the NHS in England, Wales, Scotland and Northern Ireland on External aortic root support in Marfan syndrome in 2011.
NICE updated its guidance in May 2022.
EU MDR 2017/745 conformity assessment
As a custom-made device, ExoVasc® PEARS is not eligible to carry the CE mark. However, Exstent’s Quality Management System has been audited and meets the requirements of the EU MDR 2017/745 Conformity Assessment for custom-made class III implantable devices.
US Food and Drug Administration
The ExoVasc® PEARS has not yet received clearance by the US Food and Drug Administration and is thus not available for sale in the USA at this time.
Patient information leaflets
A patient information leaflet is provided to each patient following receipt of their ExoVasc® PEARS.