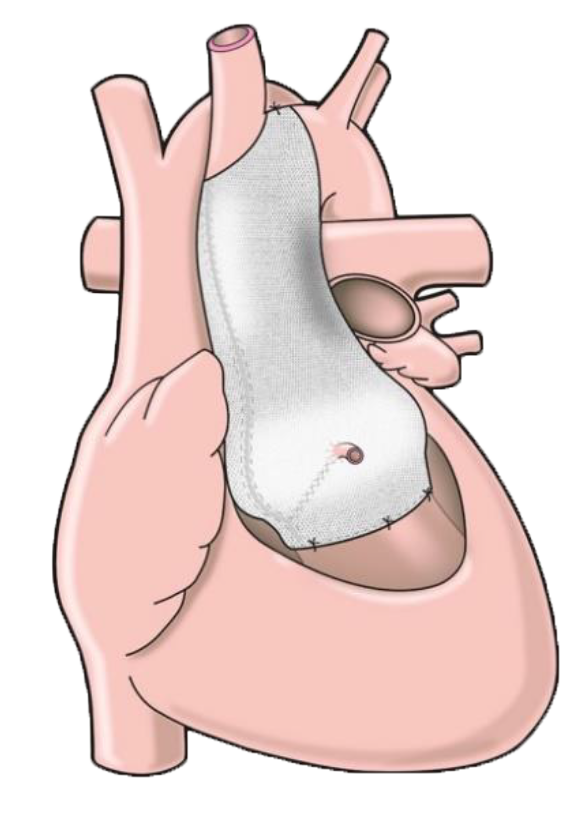
Position Description
The Development Engineer performs design and development tasks for all engineering aspects of the design and production of the ExoVasc Personalised External Aortic Root Support implant within Exstent Limited.
The Development Engineer is based within the Tewkesbury office of Exstent Limited and reports to the Research and Development Manager.
Detailed Responsibilities
- Performs design and development tasks relating to the technical performance of the ExoVasc support, the specification of the materials used in its construction, the associated verification and validation activities and maintenance of the Technical File.
- Provides technical support in the development and improvement of processes followed in all stages of the manufacture of the implant, including the performance of process validation testing.
- Maintains familiarity and contact with the surgical community worldwide to follow current trends in surgical approaches to the management of aortic dilatation and their relevance to the clinical application of the ExoVasc support.
- Reviews and collates clinical data on the use of ExoVasc for use in post market surveillance and product development activities.
- Provides technical support and training to distributors and sales staff.
- Carries out development activities associated with exploring new clinical applications for personalised external support for blood vessels and new manufacturing processes.
- Supports manufacture with the process of manipulation of patient scan data to produce resampled aortic image data suitable for computer modelling.
- Supports manufacture, with the application of the CAD modelling software and the subsequent review of the aortic CAD model.
- Provides support to the development and maintenance of software, including performing software validation to agreed plans.
- Provides technical input on the operation and maintenance of the cleanroom and the equipment used in the manufacture, packaging and sterilisation of the ExoVasc support.
- Works with the Quality and Regulatory Affairs personnel to produce Quality Management System procedures that are compliant with relevant standards, including in particular ISO 13485, FDA, and MDSAP requirements.
- Provides technical support to ensure that the operation of customer and product history files are correctly maintained
- Provides technical training to relevant personnel on all production processes.
Personnel Specification
- Educated to degree level; preferably with a mechanical or biomedical engineering or equivalent background.
- At least two years’ experience of working in industry, ideally within a medical device company including familiarity with the concepts of quality systems in medical device manufacture.
- Ability to work with exceptional attention to detail.
- Ability to communicate effectively with customers, distributors, sub-contractors and other members of the in-house team.
- Familiarity with current Information Technology and an ability to operate computer-based record systems and engineering software.
- A working knowledge of current programming languages would be advantageous.
- Enthusiasm to work in a small team and play a full part in the development of the company.